In a heart-wrenching incident that has shocked the nation, six children in Madhya Pradesh’s Chhindwara district have died within the span of three weeks due to acute kidney failure, which officials suspect was triggered by the consumption of contaminated cough syrup. The tragedy has prompted an urgent investigation by multiple government health agencies and a temporary ban on the sale of specific medications in the area.
The Tragedy Unfolds
The timeline of the deaths spans from September 4 to September 26, 2025. All six victims—children between the ages of one and seven—were reported to have consumed over-the-counter cough syrups prescribed for minor cold and flu symptoms. Initially, there was no indication of the gravity of the danger, but soon, their conditions deteriorated rapidly.
After a short period of apparent recovery, the children began to exhibit alarming symptoms such as vomiting, swelling, and anuria—the complete absence of urine output. Many were rushed to hospitals in Nagpur, a nearby city with better medical facilities. Despite receiving intensive care, including dialysis and ventilator support, six children could not be saved.
The incident set off alarm bells across the district and caught the attention of health authorities at both the state and central levels.
Suspect Syrups: Coldrif and Nextro-DS
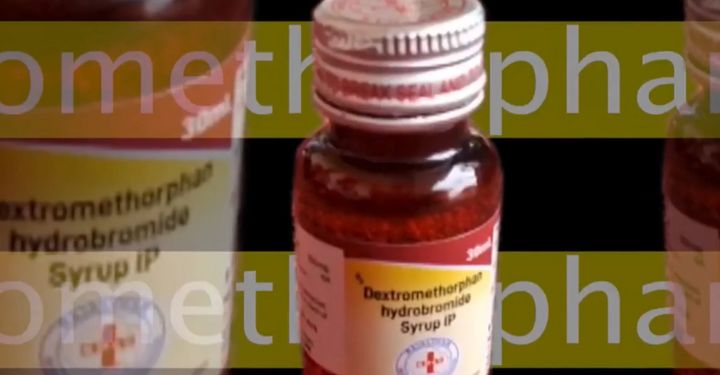
A preliminary investigation revealed that all the affected children had been administered either Coldrif or Nextro-DS, both commonly available cough syrups. Authorities wasted no time in banning the sale of these two medicines within Chhindwara as a precautionary measure.
Initial testing of drug samples, as well as kidney biopsies from the deceased, reportedly detected traces of diethylene glycol (DEG)—a toxic industrial solvent that is strictly prohibited in pharmaceutical formulations due to its known toxicity. DEG has a well-documented history of causing kidney failure and death when ingested.
Although final toxicology reports are still pending, the circumstantial and clinical evidence strongly suggests a link between the syrup and the children’s deaths.
Widening the Investigation
The incident has prompted swift intervention from the Indian Council of Medical Research (ICMR), the National Centre for Disease Control (NCDC), and teams from the Central Drugs Standard Control Organisation (CDSCO). These agencies have sent expert teams to Chhindwara to conduct on-the-ground surveillance, collect samples, and monitor the community for additional cases.
In parallel, the National Institute of Virology (NIV) in Pune and other laboratories are analyzing the contents of the syrups and biological samples from affected children. Officials are also examining other potential sources of contamination, such as local water supplies and environmental toxins, although no evidence has yet been found outside the medication link.
Authorities are conducting door-to-door screening in affected villages, particularly in the Harrai block, where most of the cases originated. Parents are being advised to seek immediate medical care if their child has a fever lasting more than one day or shows signs of urinary retention.
A Pattern with Precedent
This tragedy is the latest in a series of cough syrup-related poisoning incidents that have emerged in recent years, both in India and abroad. In 2022 and 2023, similar cases were reported in The Gambia, Uzbekistan, and Cameroon, where cough syrups containing DEG led to the deaths of dozens of children. Many of those syrups were traced back to manufacturers based in India, leading to growing international scrutiny.
The World Health Organization (WHO) has previously issued multiple alerts about cough syrup safety, urging member states to strengthen their regulatory oversight and quality control mechanisms.
Now, as this crisis hits home once again, experts are calling for stricter domestic regulation and immediate enforcement of safety protocols in the pharmaceutical supply chain.
Medical Community on Alert
Doctors in the region have been advised to stop prescribing combination syrups, particularly to children, and instead opt for targeted symptom relief—such as using paracetamol for fever or saline nasal drops for congestion. Health officials are also urging the public to avoid self-medication and to seek medical advice before giving any over-the-counter drugs to children.
An internal advisory from the Union Health Ministry has directed all states and union territories to promote the “rational use of syrups in pediatric populations”, particularly where the efficacy of such combinations is unproven.
Families Seek Answers
As grieving families prepare to bury their children, they are demanding justice and accountability. Questions are being raised about how contaminated syrups ended up in the supply chain and who failed to catch the problem before lives were lost.
“I trusted the medicine prescribed to my daughter, never imagining that it would be the very thing that took her from us,” said the father of a 3-year-old victim from Harrai village. “Now I want answers, and I want the people responsible to face justice.”
Local politicians and civil society organizations are calling for compensation, criminal investigation, and long-term reforms in how medicines are tested, approved, and distributed—particularly in rural and semi-urban areas where oversight is often weaker.
Policy Implications and the Road Ahead
This tragedy may serve as a critical turning point in India’s public health and pharmaceutical regulation landscape. While India is often referred to as the “pharmacy of the world” due to its vast drug manufacturing industry, this status also comes with the responsibility to maintain global safety standards.
Experts are calling for:
- Mandatory batch testing of pediatric medications before release
- Transparent recall systems for potentially harmful products
- Greater investment in rural drug regulation infrastructure
- Training programs for local doctors and pharmacists to recognize early signs of drug-induced toxicity
Conclusion
The deaths of six innocent children in Chhindwara have cast a grim spotlight on the dangers of unchecked medication use and lax regulatory enforcement. As investigations continue, this incident underscores the urgent need for systemic reform and renewed vigilance to prevent another such tragedy.




More Stories
Despite Millions Spent, Delhi’s Cloud-Seeding Trial Yields No Rain
Raped, Silenced, and Pushed to the Edge: Young Doctor’s Final Note Names MP and Police Officials
Voter List Fraud: ₹80 Offered for Each Fake Deletion Request in Aland, Karnataka SIT Reveals